Chemical change –
one or more new substances with new physical and chemical properties are formed.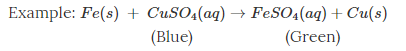
Here,
when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper, are formed.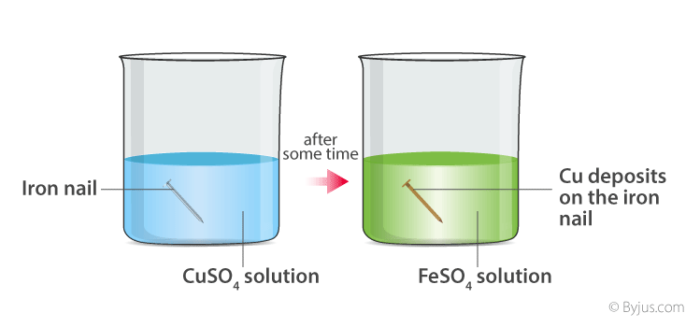
Physical
change – change in colour or state occurs, but no new substance is formed.
Example: Water changes to steam on boiling, but no new substance is
formed (Even though steam and water look different when they are made to react with a piece of Na, they
react the same way and give the exact same products). This involves only a change in state (liquid to
vapour).
To know more about Physical and Chemical Changes, visit here.
Students can refer to the short notes and MCQ questions along with separate solution pdf of this chapter for quick revision from the links below:





